45 what does exempt human specimen mean
PDF QUICK GUIDE - Using Human Biological Specimens at UC Davis identifiers linked to the specimens. If the investigator does not record the identifiers or link them to the specimens, the research may be eligible for review under the exempt category. Specifically, exempt category #4 applies to research that involves the collection or study of existing* data, What is Exempt | NYU Wagner Research involving the collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens, if these sources are publicly available or if the information is recorded by the investigator in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects.
Lesson 2: What is Human Subjects Research? | HHS.gov This is commonly referred to as non-exempt human subjects research. Note that, in addition to the Common Rule (subpart A), non-exempt human subjects research funded by HHS must also comply with subparts B, C, & D of the regulations at 45 CFR 46. These subparts provide additional protections for certain special populations involved in research.
What does exempt human specimen mean
Human Subjects Research - Home page | grants.nih.gov Feb 28, 2019 · Learn more about research that meets the definition human subjects research, Federal regulation requirements, and whether your project may be considered exempt. Also, learn about NIH-specific considerations and become more familiar with NIH policies, and other regulations as it relates to human subjects research protections. Travellers and online shoppers - DCCEEW If you buy something locally, it does not necessarily mean you can take it overseas. Don't be fooled by statements like: "Believe me, it's OK". If you intend to buy or travel with wildlife products, contact the appropriate government departments before you leave or enter a country to find out if you need a permit. Without the correct permit, your product maybe seized by border control ... Boxes - Sarstedt What do P650 "light" and "exempt human specimen" mean? This is an exemption under category B (infectious substances allocated UN3373), but only applies if there is a professional assessment (e.g. from a doctor) stating that the patient samples to be shipped have no or minimal likelihood of containing a pathogen.
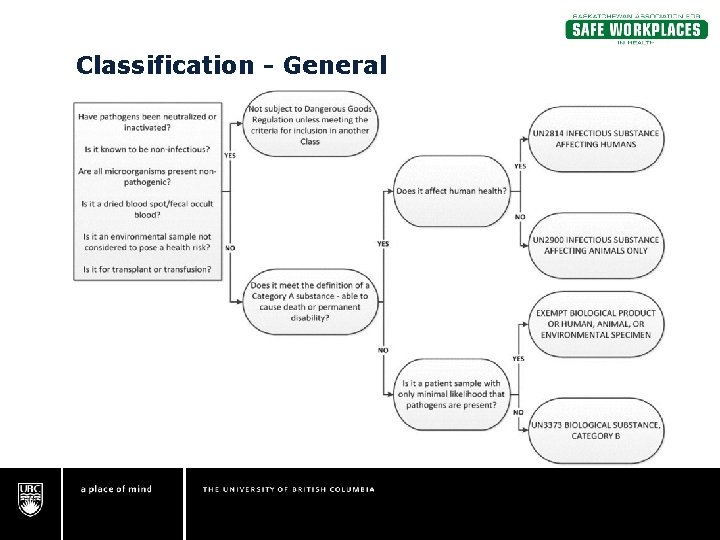
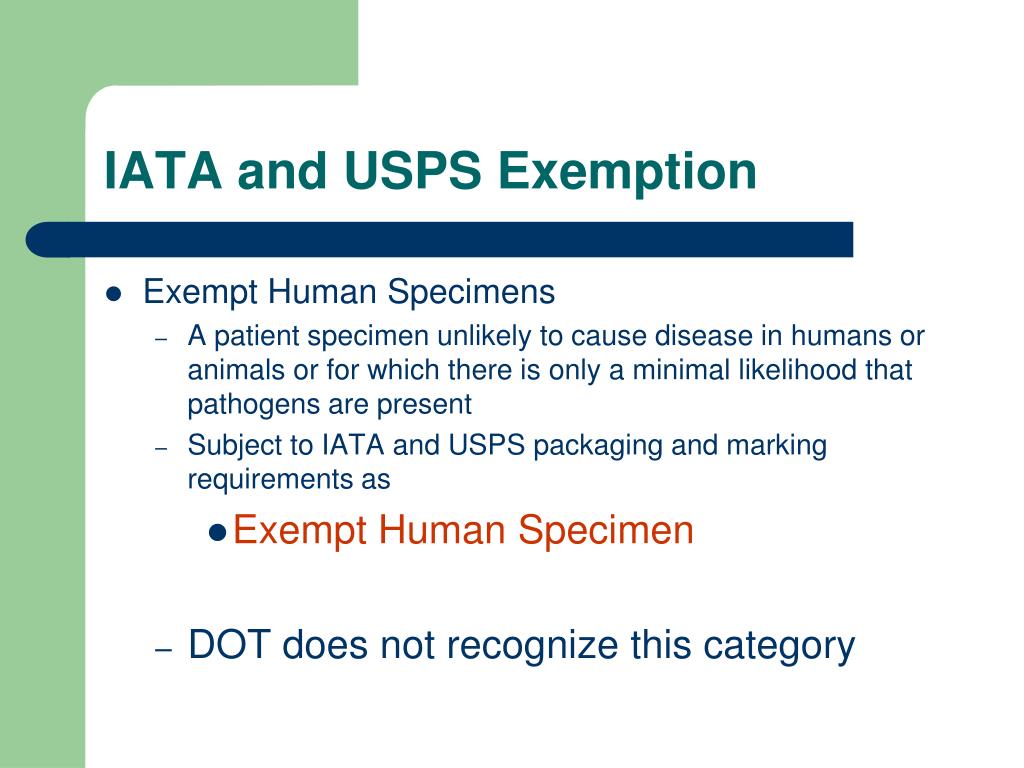
What does exempt human specimen mean. What does the term "exempt" actually mean in human subjects research ... Human subjects research that is classified as "exempt" means that the research qualifies as no risk or minimal risk to subjects and is exempt from most of the requirements of the Federal Policy for the Protection of Human Subjects, but is still considered research requiring an IRB review for an exemption determination. PDF Exempt Human Specimen / Exempt Animal Specimen Reference Guide (IATA 3 ... Package is marked with the words "Exempt human specimen" or "Exempt animal specimen", as appropriate. (this would be in lieu of a UN3373 label). 2. The packaging must consist of three components: a. a leak-proof primary receptacle(s); b. a leak-proof secondary packaging; and Guidelines for Safe Work Practices in Human and Animal ... Jan 06, 2012 · Ensure that specimen placement, specimen flow, and bench operational workflow are unidirectional (i.e., from clean areas to dirty areas) and uniform for all operators to maximize effective use of engineering controls. Determine appropriate PPE on the basis of documented risk and hazard assessments of all the operations performed at each bench. Definitions | ORI - The Office of Research Integrity - HHS.gov research and demonstration projects which are conducted by or subject to the approval of department or agency heads; or. taste and food quality evaluation and consumer acceptance studies. It is critically important to note, however, that decisions about whether studies are exempt from the requirements of the Common Rule must be made by an IRB ...
Human Subjects Research - Home page | grants.nih.gov 28/02/2019 · Learn more about research that meets the definition human subjects research, Federal regulation requirements, and whether your project may be considered exempt. Also, learn about NIH-specific considerations and become more familiar with NIH policies, and other regulations as it relates to human subjects research protections. Job Status: Exempt vs. Non-Exempt | Human Resources | Georgia Institute ... These classifications are based on several factors such outlined in the federal regulations and Board of Regents rules as the actual job duties outlined in the job description, as well as the salary. Exempt Employee Not eligible for overtime Must record exceptions to work (sick, vacation, jury duty, bereavement, etc.) Non-Exempt Employee Definition of Human Subjects Research | grants.nih.gov According to 45 CFR 46 , a human subject is "a living individual about whom an investigator (whether professional or student) conducting research: Obtains information or biospecimens through intervention or interaction with the individual, and uses, studies, or analyzes the information or biospecimens; or Study Risk Levels | Rutgers Research Exempt Review means the research falls under the 8 categories of human subjects research that are exempt from the other provisions of the regulations. The regulations found in 45 CFR 46.101 have determined that the following eight categories of research are eligible for exemption status, [45 CFR 46.101(b)]: This review type does NOT mean that ...
Lesson 2: What is Human Subjects Research? | HHS.gov Human subjects research studies that do not qualify for an exemption are referred to as non-exempt human subjects research. Unless there is a Secretarial waiver, they must comply with the Common Rule requirements, including IRB review and approval, before the research can begin. For non-exempt cooperative research studies involving multiple institutions, the review … PDF IRB definitions (Is it Research? And definitions of exempt, expedited ... Priority concern is research using human subjects, which is designed, conducted, supervised or directed by a faculty member. All research studies using human subjects are required to have some level of review (Even exempt studies need to be reviewed- at the departmental level or by the IRB). Exempt does not mean "no review"! What is "Exempt" Human Subject Research, And What Does It Mean? (2019 ... WHAT IS "EXEMPT" HUMAN SUBJECTS RESEARCH, AND WHAT DOES IT MEAN? Briefly, research is termed "Exempt" when it constitutes research with human subjects, but ALSO meets the requirements of a defined low-risk category that is exempt from SOME (but not all) of the requirements governing human subjects research. To qualify as Exempt, the research must: Exempt Categories | Human Research Protection Program | Michigan State ... Exempt 4. Collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens if publicly available or information is recorded by investigator in a manner that subjects cannot be identified. Exempt 5. Federal demonstration projects. Exempt 6. Taste and food quality evaluation and consumer acceptance studies.
PDF 1 Meets the definition of human subjects research. specimens if publicly available, or recorded such that subjects cannot be identified* *May be identifiable in limited cases. See §46.104(d)(4)(iii) and (iv) Exemption 5: public service program research or demonstration projects Exemption 6: taste and food quality evaluations Exemption 7: storage of identifiable information or
What is climate change and why does it matter? Find out what climate change is, why it matters and what it could mean for our collective future. What are weather, climate and climate change? Weather refers to atmospheric conditions, such as rain or snow, happening in a place at a specific moment in time. Climate is how much, on average, a type of weather will occur over a longer period.
ASPAG - Overview: Aspergillus (Galactomannan) Antigen, Serum The presence or absence of Aspergillus galactomannan antigen in the test sample is determined by calculation of an index for the specimen. The index is the optical density (OD) value of the specimen divided by the mean OD of wells containing the cutoff control serum (low-positive control).(Package insert: Platelia Aspergillus EIA.
IRB FAQs for Survey Researchers - AAPOR According to the Federal regulations (45 CFR 46.101(b)), survey research may be exempt from the regulations unless "the information obtained is recorded in such a manner that the human subjects can be identified, directly or through identifiers linked to the subjects" or if "federal statute(s) require(s) without exception that the ...
Exempt patient specimens - un3373.it EXEMPT HUMAN SPECIMEN or EXEMPT ANIMAL SPECIMEN They are collected directly from humans or animals and there is minimal likelihood that pathogens are present. An element of professional judgment is required to determine if a substance is exempt under this paragraph.
What is climate change and why does it matter? | Natural ... Differing rates of change could mean that species' lives are no longer synchronised with those they rely on. Many plants are flowering earlier. Migrating birds arrive earlier, leave later and some even are getting smaller. Butterflies are emerging earlier. Birds and amphibians are laying their eggs earlier in the year.
PDF Proper Shipment of Patient Specimens and Infectious Substances Patient Specimens (minimal likelihood that pathogens are present) N/A N/A N/A See Checklist on Pg.6 Exempt Human Specimen or Exempt Animal Specimen N/A N/A N/A Non-infectious specimens (mammals, birds, amphibians, reptiles, fish, insect and other invertebrates) containing small quantities of flammable preservative
ASPAG - Overview: Aspergillus (Galactomannan) Antigen, Serum The presence or absence of Aspergillus galactomannan antigen in the test sample is determined by calculation of an index for the specimen. The index is the optical density (OD) value of the specimen divided by the mean OD of wells containing the cutoff control serum (low-positive control).(Package insert: Platelia Aspergillus EIA.
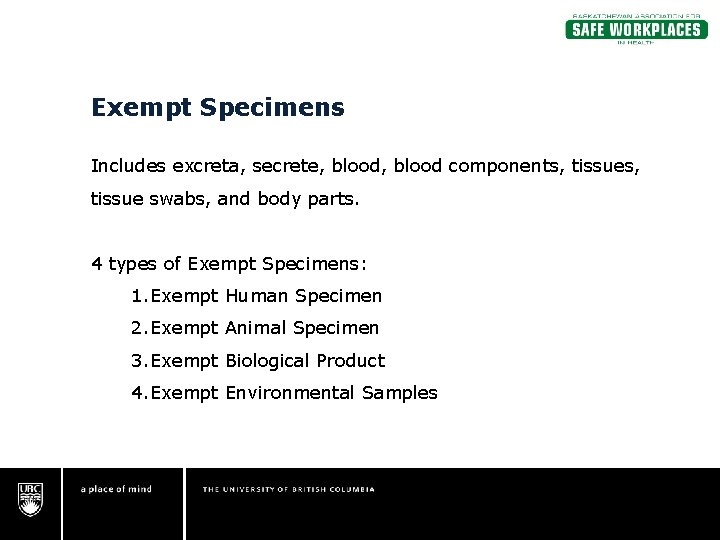
USPS Packaging Instruction 6H | Postal Explorer "Exempt human or animal specimen" means a human or animal sample (including, but not limited to, secreta, excreta, blood and its components, tissue and tissue fluids, and body parts) transported for routine testing not related to the diagnosis of an infectious disease.
Waived Tests | CDC Waived tests include test systems cleared by the FDA for home use and those tests approved for waiver under the CLIA criteria. Although CLIA requires that waived tests must be simple and have a low risk for erroneous results, this does not mean that waived tests are completely error-proof. Errors can occur anywhere in the testing process ...
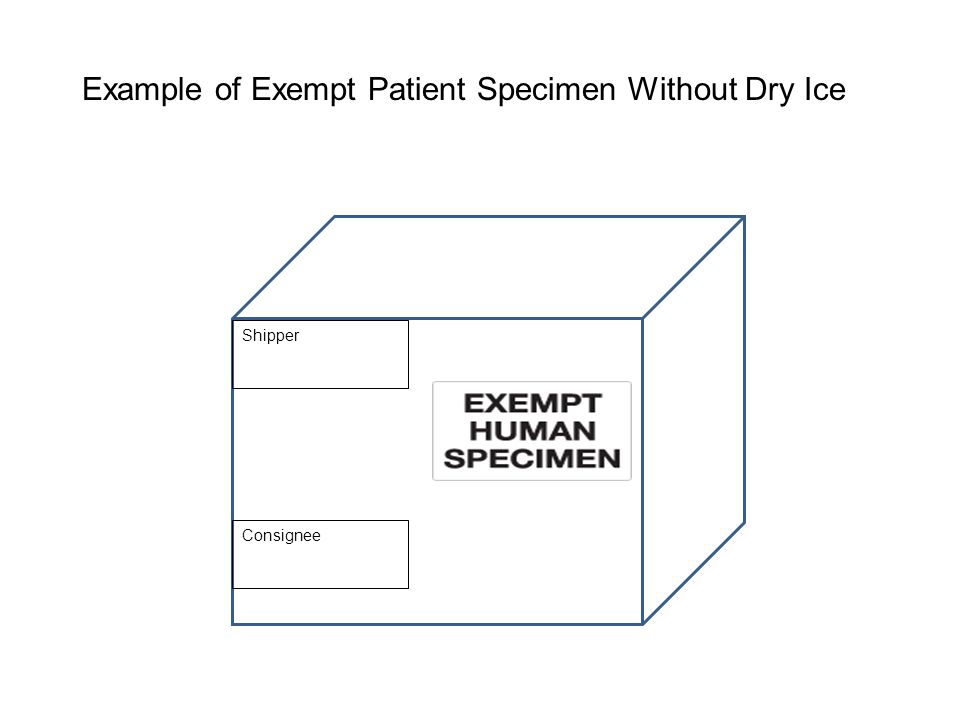
How to Ship Clinical Samples | FedEx Liquid clinical samples marking requirements Include a marking on the package that properly identifies the shipment as "Exempt Human Specimen" or "Exempt Animal Specimen" as appropriate to comply with current IATA and ICAO regulations. If you prefer, package markings may be in the form of a label. Back To Top Ready to ship? Create a shipment online
Shipping Infectious Substances - Transport Canada In this case, you may ship the sample as "Exempt Human Specimen" if the medical professional has no reason to believe that the person has been in contact with an infectious substance. Examples of specimens that may be transported under this section include: Blood or urine specimens to monitor cholesterol levels, blood sugar levels or hormone ...
Exempt Research Definition | Office of Research Oversight/Regulatory ... Categories of exempt research are stipulated in Federal regulations at 45 CFR46.101(b)(1-6) as follows: (i.) Research conducted in established or commonly accepted educational settings, involving normal educational practices, such as(i) research on regular and special education instructional strategies,or (ii) research on the effectiveness of or the comparison among instructional techniques ...
Attachment C - Updated FAQs Informed Consent for Use of Biospe While it is true that permanently stripping a specimen of all identifiers or codes would mean that subsequent uses are not considered to be human subjects research, doing this after the subject has made the request for withdrawal of the specimens would be ethically suspect, if done solely to avoid withdrawing specimens on request. If the specimen is identifiable at the time of the …
FAQs: Bloodstream Infection (BSI) Events | NHSN | CDC The collection site (venipuncture site or central line drawn) of the blood specimen does not determine the eligibility of the positive blood specimen to meet CLABSI criteria. All positive blood specimens, regardless of the sites from which they were collected or the purposes for which they were drawn must be included in CLABSI surveillance. NHSN does not require that a BSI be …
PDF EXEMPT RESEARCH - University of California, Berkeley This guidance document is intended for researchers planning to carry out minimal-risk activities with human subjects, which may qualify as "exempt" from federal regulatory requirements. Should you need additional assistance, please contact OPHS at 510-642-7461 or ophs@berkeley.edu. Table of Contents: A.General Information
Product - Sarstedt What do P650 "light" and "exempt human specimen" mean? This is an exemption under category B (infectious substances allocated UN3373), but only applies if there is a professional assessment (e.g. from a doctor) stating that the patient samples to be shipped have no or minimal likelihood of containing a pathogen.
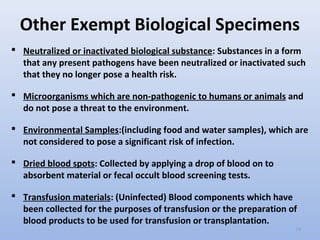
Frequently Shipped Biological Material and Proper Classification Professional judgment must be used; if you suspect the specimen may contain an infectious substance, it must be shipped accordingly. These shipments should be packaged using a triple packaging system and marked as "exempt human specimen" or "exempt animal specimen." Exempt patient specimens include: Biopsies. Dried blood spots.
EUR-Lex - 32019R1009 - EN - EUR-Lex - Europa In order to minimise risks to human health, to safety or to the environment that may be posed by polymers other than nutrient polymers, the criteria for their biodegradability, so that they are capable of undergoing physical and biological decomposition, should be established. For that purpose, the power to adopt acts in accordance with Article 290 TFEU should be delegated to …
Domestic Mail - USPS Exempt human or animal specimen means a human or animal sample (including, but not limited to, secreta, excreta, blood and its components, tissue and tissue fluids, and body parts) transported for routine testing not related to the diagnosis of an infectious disease. Typically, exempt human specimens are specimens for which there is a low ...
Attachment B - Recommendations | HHS.gov the exemption is an acknowledgment that a subset of research activities that are already protected by hipaa—secondary research involving protected health information ("phi")—already afford human subjects rigorous regulatory protection of their privacy and that aside from privacy risks, these activities typically have lower overall human subjects …
45 CFR 46 | HHS.gov The HHS regulations for the protection of human subjects in research at 45CFR 46 include five subparts. Subpart A, also known as the Common Rule, provides a robust set of protections for research subjects; subparts B, C, and D provide additional protections for certain populations in research; and subpart E provides requirements for IRB registration.
Category B - UN3373.com -Human or animal specimens for which there is minimal likelihood that pathogens are present are not subject to these Regulations if the specimen is transported in a packaging which will prevent any leakage and which is marked with the words "Exempt human specimen" or "Exempt animal specimen", as appropriate.
Guidelines for Safe Work Practices in Human and Animal … 06/01/2012 · All functions of the human and animal diagnostic laboratory — microbiology, chemistry, hematology, and pathology with autopsy and necropsy guidance — are addressed. A specific section for veterinary diagnostic laboratories addresses the veterinary issues not shared by other human laboratory departments. Recommendations for all laboratories include use of …
PDF The ABCs of 104: Understanding Exemption Categories - HHS.gov • the study meets the regulatory definition for human subjects researchbut satisfies the conditions for one or more of the eight exempt categories described in the common rule • exempt studies are excused from the typical requirements of the common rule, such as, irb review according to the criteria at 46.111 and the informed consent requirements …
What Is A Human Specimen Definition - WhatisAny "Exempt human or animal specimen" means a human or animal sample (including, but not limited to, secreta, excreta, blood and its components, tissue and tissue fluids, and body parts) transported for routine testing not related to the diagnosis of an infectious disease. What does 3373 mean?
IRB FAQs for Survey Researchers - AAPOR One might think that exempt status would give the investigator complete freedom over the conduct of the research, but the IRB retains an interest in the exempt study and at least some authority over its conduct. At present, there seems to be considerable local variation in the amount of control exercised by the IRB over an exempt study and, conversely, the amount of …
Boxes - Sarstedt What do P650 "light" and "exempt human specimen" mean? This is an exemption under category B (infectious substances allocated UN3373), but only applies if there is a professional assessment (e.g. from a doctor) stating that the patient samples to be shipped have no or minimal likelihood of containing a pathogen.
Travellers and online shoppers - DCCEEW If you buy something locally, it does not necessarily mean you can take it overseas. Don't be fooled by statements like: "Believe me, it's OK". If you intend to buy or travel with wildlife products, contact the appropriate government departments before you leave or enter a country to find out if you need a permit. Without the correct permit, your product maybe seized by border control ...
Human Subjects Research - Home page | grants.nih.gov Feb 28, 2019 · Learn more about research that meets the definition human subjects research, Federal regulation requirements, and whether your project may be considered exempt. Also, learn about NIH-specific considerations and become more familiar with NIH policies, and other regulations as it relates to human subjects research protections.

















Post a Comment for "45 what does exempt human specimen mean"