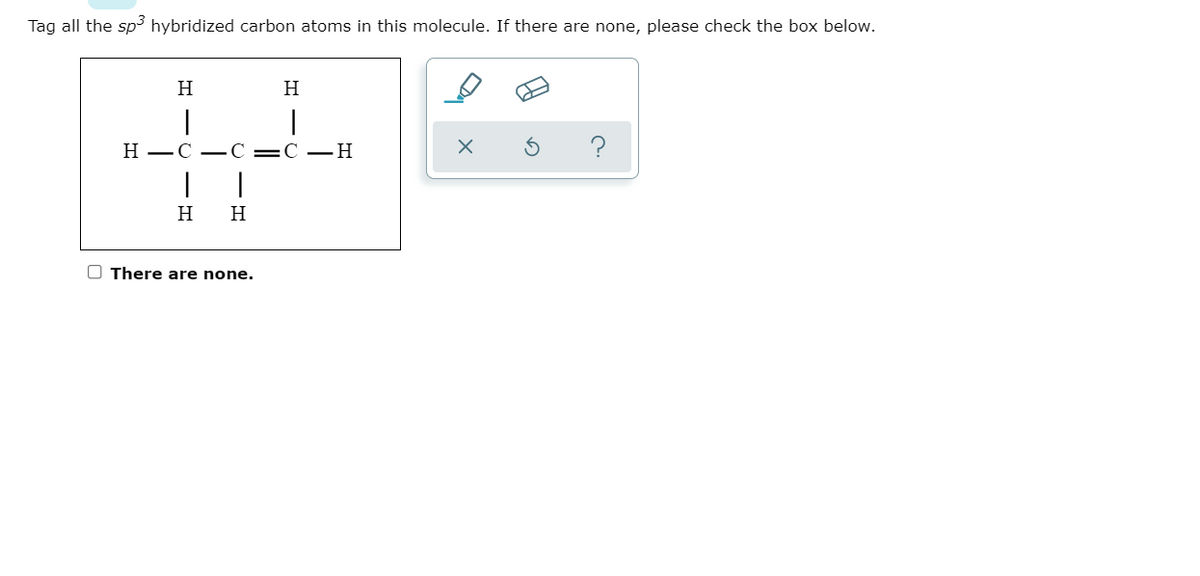
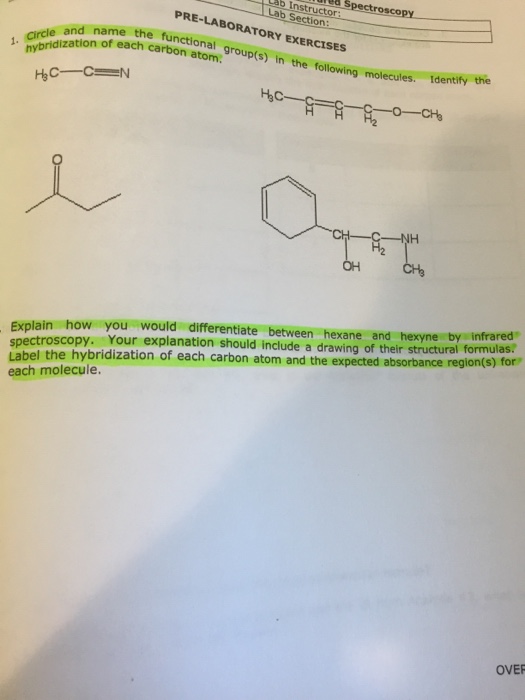
44 label each carbon atom with the appropriate hybridization.
Solved Label each carbon atom with the appropriate | Chegg.com Question: Label each carbon atom with the appropriate hybridization. This problem has been solved! ... Label each carbon atom with the appropriate hybridization. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. What is the hybridization of the carbon atom labelled 2? It is s p hybrid because the labelled 2 carbon has C − C triple bond, and it has 2 sigma bonds, and zero lone pairs, hence, it has s p hybridization. Two sp hybridized orbitals: The chemical bonding in compounds such as alkynes with triple bonds is explained by s p hybridization.
Hybridization of Carbon - Molecular Geometry and Bond Angles - BYJUS A carbon atom is sp2 hybridized when bonding takes place between 1 s-orbital with two p orbitals. There is a formation of two single bonds and one double bond between three atoms. The hybrid orbitals are placed in a triangular arrangement with 120° angles between bonds. Example: Hybridization of graphite 3. sp3 Hybridization

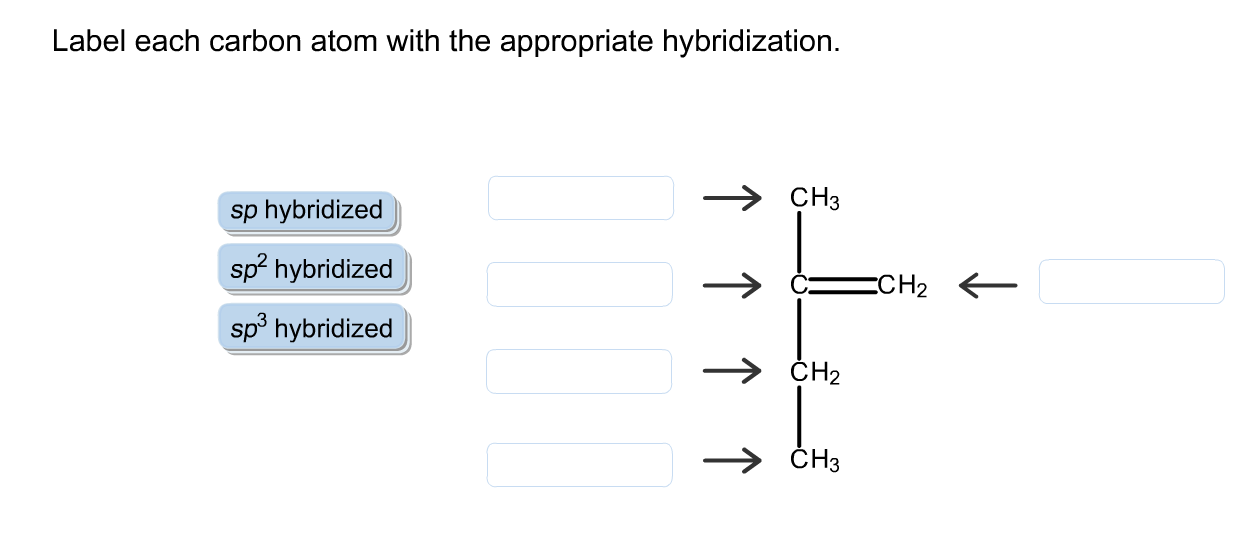
Label each carbon atom with the appropriate hybridization.
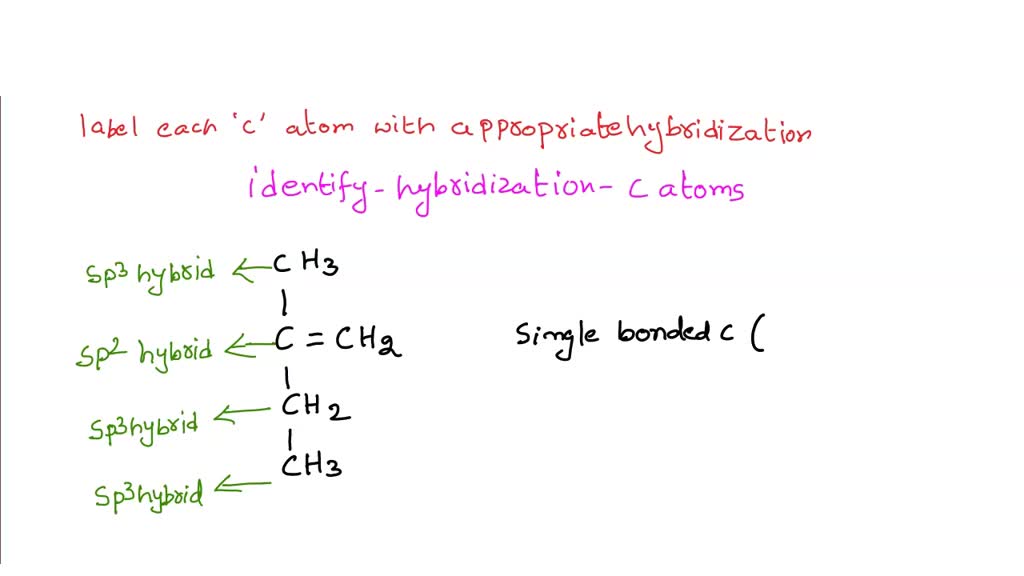
Can you label each carbon atom with the appropriate hybridization .... C=C bonded C atoms are planar and sp2 hybridized. C-C bonded C are sp3 hybridized. In this compound one C=C present and hence both carbons are sp2 hybridized. Left all the C atoms have four single bond around each C atom. so left all the C atoms are sp3 hybridized. number of electrons pairs around sp3 hybridized C atoms = 4 and PDF The Hybridization Model of Atoms in Molecules - cpp.edu the carbon atoms, perpendicular to the plane of the page. On the other side of each carbon atom, 180o away from the other carbon atom, we can attach a simple hydrogen atom, using its 1s atomic orbital to overlap in a sigma bond along the bonding axis (a first bond is always sigma bond). C C σCC H C σCH C H σCH C C πCC πCC C C top and ... label each carbon atom with the appropriate hybridization 🚀To book a personalized 1-on-1 tutoring session:👉Janine The Tutorhttps://janinethetutor.com🚀More proven OneClass Services you might be interested in:👉One...
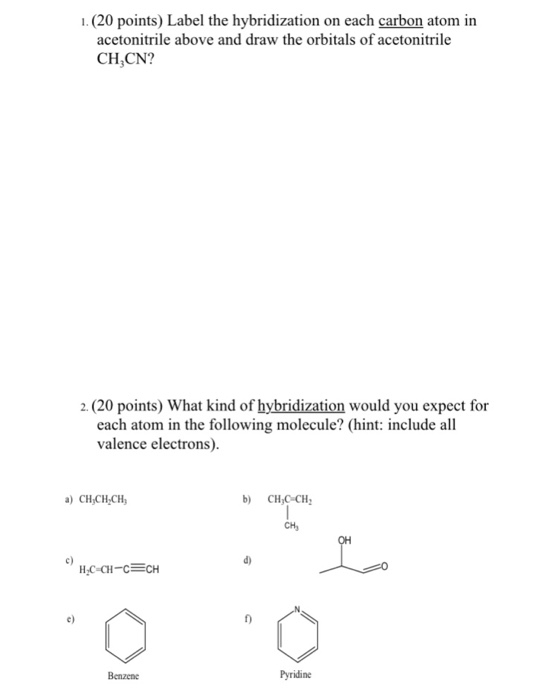
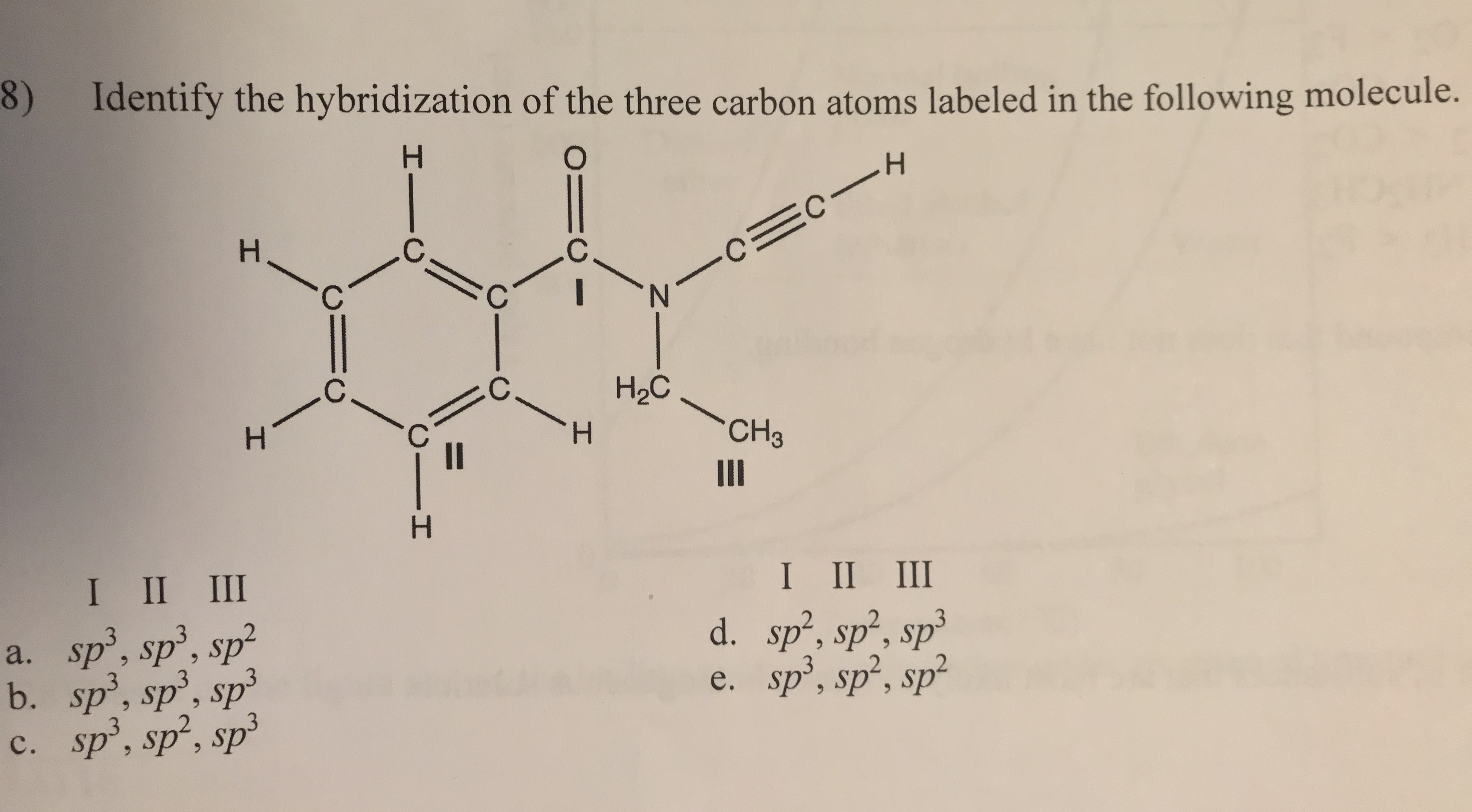
Label each carbon atom with the appropriate hybridization.. Finding the hybridization of atoms in organic molecules (worked ... And so, the fast way of identifying a hybridization state, is to say, "Okay, that carbon has "a double bond to it; therefore, it must "be SP two hybridized." And if it's SP two hybridized, we know the geometry around that carbon must be trigonal, planar, with bond angles approximately 120 degrees. Solved Label each carbon atom with the appropriate | Chegg.com Label each carbon atom with the appropriate hybridization. Question: Label each carbon atom with the appropriate hybridization. This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text Expert Answer. Who are the experts? Chapter 9 Homework Flashcards - Questions and Answers | Quizlet There are 6 C atoms in the molecule. Starting on the left, the hybridizations are: sp2, sp2, sp3, sp, sp, sp3. All single bonds are bonds. Double and triple bonds each contain 1 bond. This molecule has 8 C-H bonds and 5 C-C bonds, for a total of 13 bonds. Double bonds have 1 bond and triple bonds have 2 bonds. This molecule has a total of 3 bonds. What is the hybridization of each carbon atom in acetonitrile? The total number of valence electrons present in a molecule of acetonitrile will be equal to 16 because you have. 2 × 4 e− = 8 e− → from two atoms of carbon, C. 3 × 1 e− = 3 e− → from three atoms of hydrogen, H. 1 × 5 e− = 5 e− → from one atom of nitrogen, N. Now, the two carbon atoms will be bonded together via a single bond.
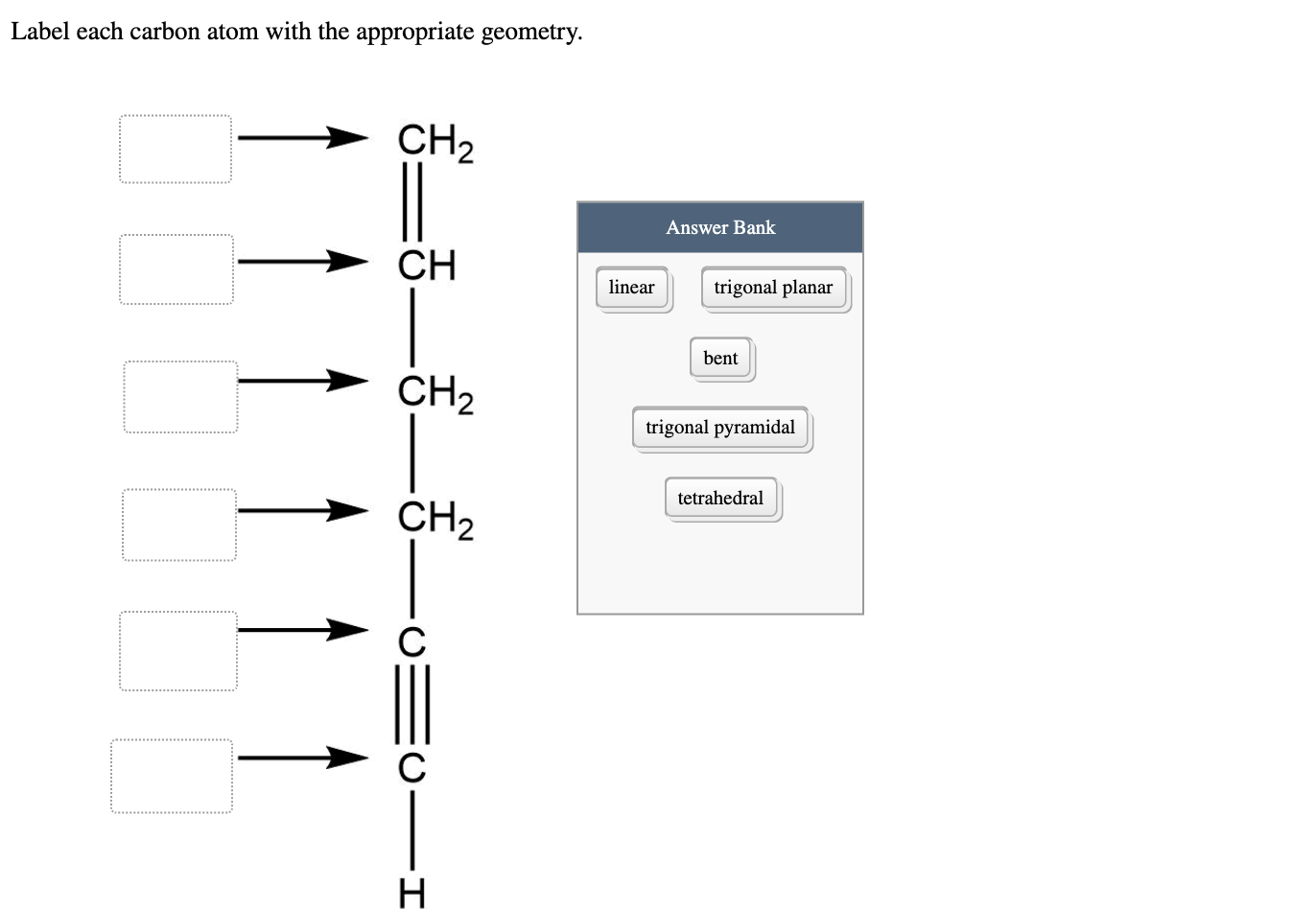
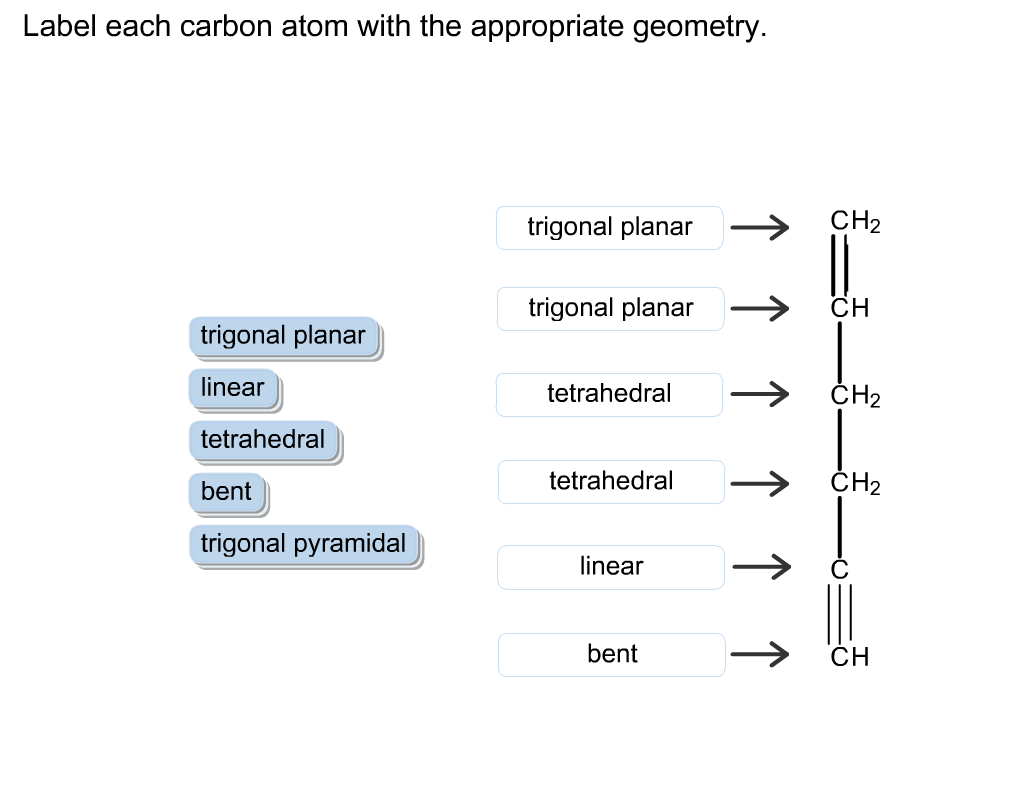
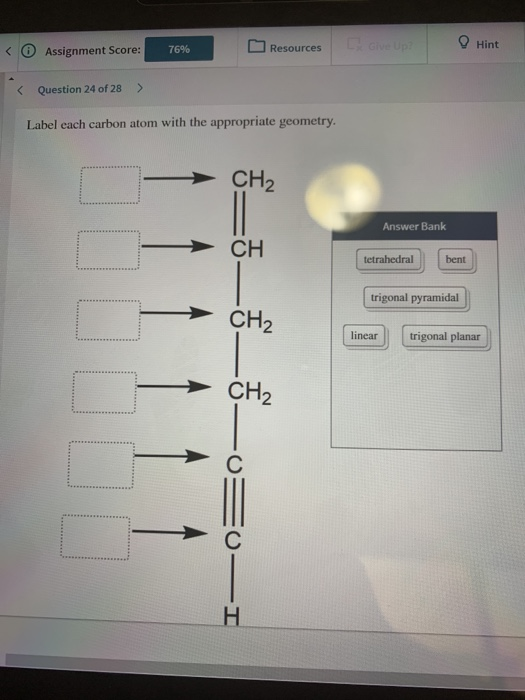
Answered: Label each carbon atom with the… | bartleby Solution for Label each carbon atom with the appropriate geometry. CH2 Answer Bank CH tetrahedral linear trigonal planar bent CH2 trigonal pyramidal CH2 C C H. ... Predict the approximate molecular geometry around each carbon atom of acetonitrile. H3C-C=N: The… A: When carbon is attached by 4 single bond with other atom , then the ... Solved Label each carbon atom with the appropriate | Chegg.com Expert Answer 100% (7 ratings) Pi bonds are … View the full answer Transcribed image text: Label each carbon atom with the appropriate hybridization. sp hybridized > CH3 > -CH2 { spr hybridized sp3 hybridized * * * * > CH2 > CH3 Previous question Next question New questions in Chemistry - Brainly.com The electrons of each carbon atom are found in one s-orbital and three p-orbitals. However, when forming sigma bonds, the carbon atoms combine the four atomic orbitals into four molecular orbitals. This results in each carbon now having four hydridized sp3 orbitals. Therefore, each carbon atom is sp3 hybridized. 'Label each carbon atom with the appropriate hybridization. 9p ... Therefore, electron pairs surrounding each peripheral carbon four electron pairs is always SP three hybridized or four electron groups. Here we have three electron groups, two of them single bonds, one of them double bonds. Three electron groups corresponds to SP two hybridized. David C. Weber State University Chemistry: An Atoms-Focused Approach
Answered: What is the hybridization at each… | bartleby Q: The hybridization of the carbon atom labeled x in the molecule below is H н :0: х H-N-C-cö-H н—с—н O… A: Hybridization is the mixing of the atomic orbital of same symmetry and similar energy to give rise… Label each carbon atom with the appropriate geometry. - Transtutors Label each carbon atom with the appropriate geometry. Two binary symmetric channels (BSC) are connected in cascade as shown below. input — BSC BSC 2 output 1 Both the channels have the same transition probability and the error/cross over probability... Posted one year ago Recent Questions in Math Q: [Solved] Label each carbon atom with the appropriategeometry ... Important Concepts and reason. Before overlapping with 1s orbital of hydrogen, first, the atomic orbitals of carbon undergoes hybridization to form hybrid orbitals. The hybrid orbitals of carbon involve in bond formation with hydrogen. Hence, the geometry at each carbon depends on the type of hybridization. OneClass: Label each carbon atom with the appropriate hybridization.sp Get the detailed answer: Label each carbon atom with the appropriate hybridization.sp,sp2 or sp3 Label each carbon atom with the appropriate hybridization.
What are hybridisation states of each carbon atom in the following ... The hybridisation states of each carbon atom in the following compounds are given below. ... sp2 Hybridization. 10 mins. sp3, sp3d and sp3d2 Hybridization. 22 mins. sp3d3 Hybridization. 11 mins. Shortcuts & Tips . Mindmap > Common Misconceptions > Cheatsheets > Important Diagrams > Problem solving tips >
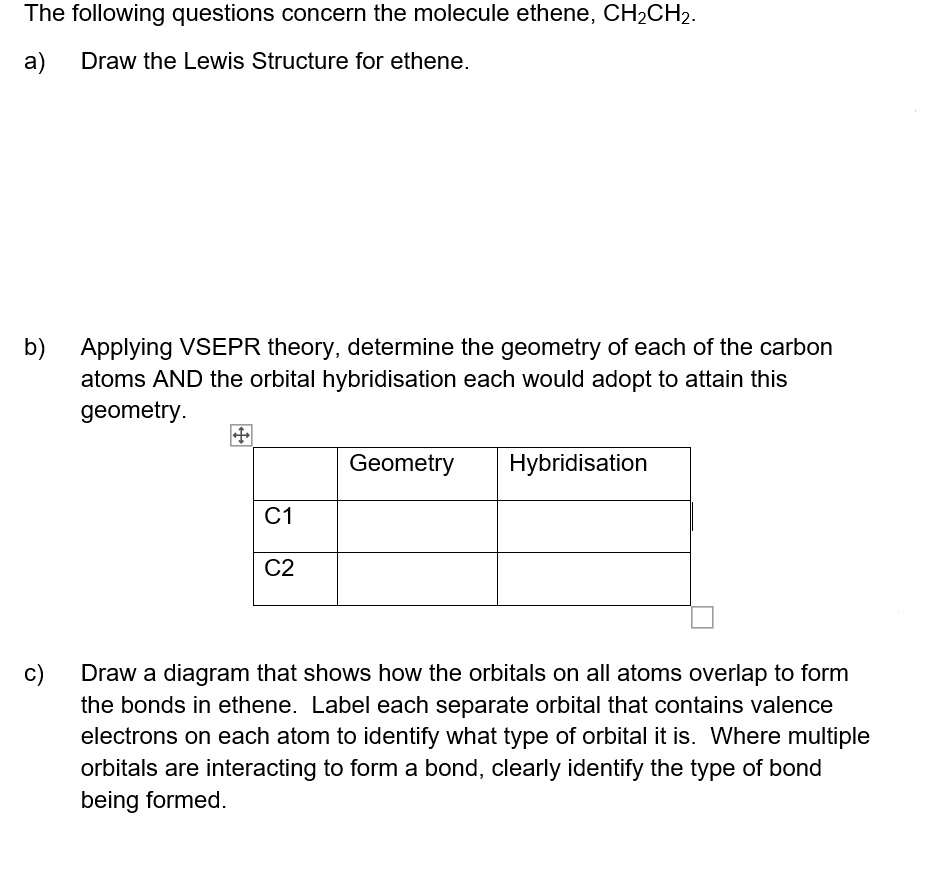
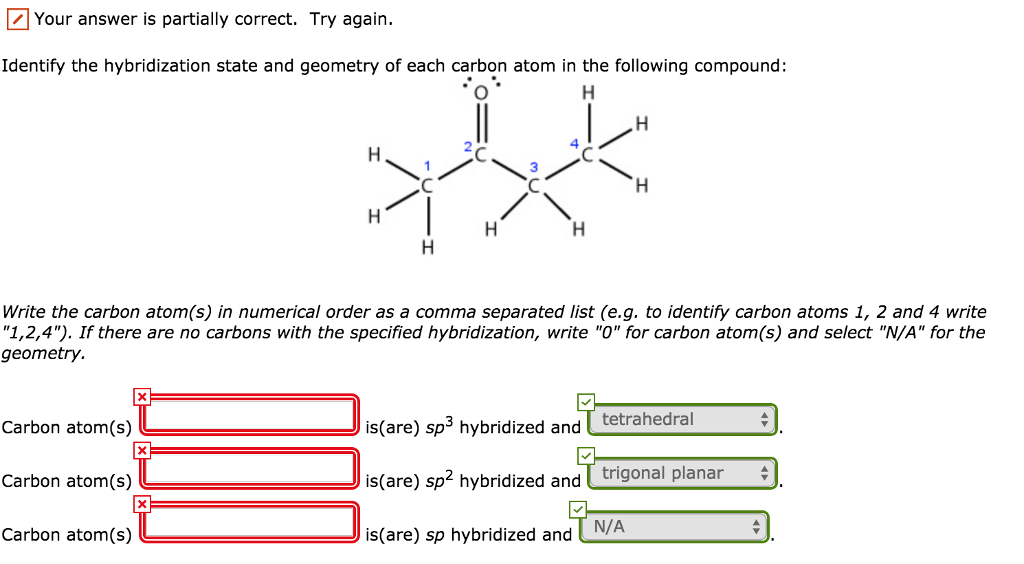
Label each carbon atom with the appropriate geometry. Label each carbon ... Label each carbon atom with the appropriate geometry. Answer Concepts and Reason Before they overlap with the 1st orbital of hydrogen, the first atomic orbitals undergo hybridization to create hybrid orbitals. Hybrid orbitals of carbon are formed through hydrogen bond formation. The type of hybridization determines the geometry at each carbon.
Quiz1 88%.docx - Question 1 Label each carbon atom with the appropriate ... View Quiz1 88%.docx from CHEM 2301K at Albany State University. Question 1 Label each carbon atom with the appropriate hybridization. Question 2 Determine the formal charge on each atom in the
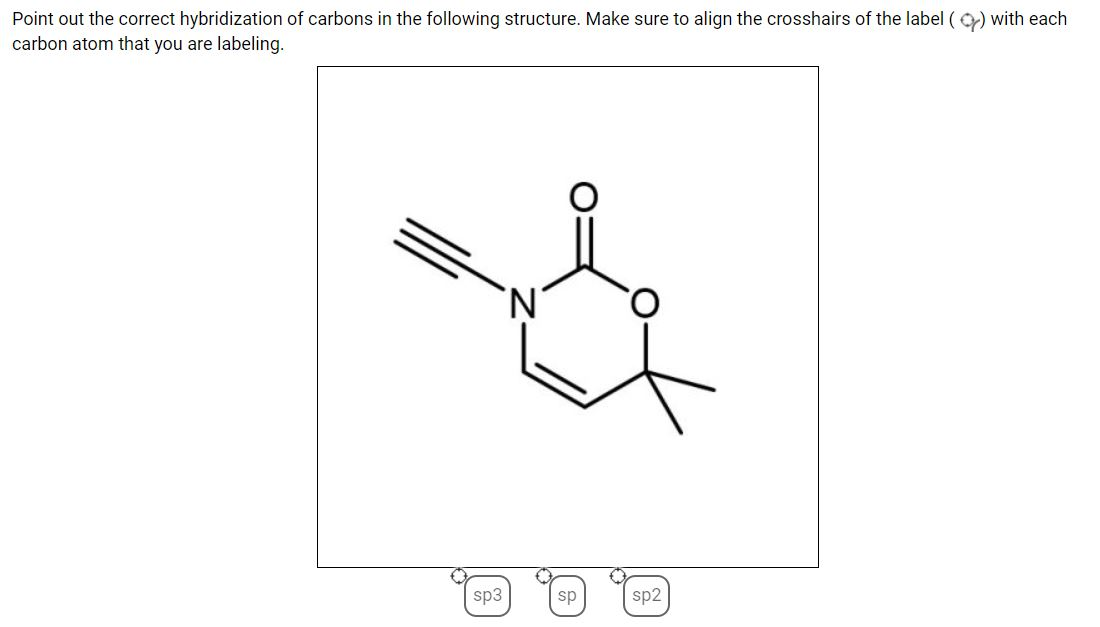
Hybridization - sp, sp2, sp3, sp3d, sp3d2 Hybridized Orbitals, Examples sp hybridization is also called diagonal hybridization. Each sp hybridized orbital has an equal amount of s and p character - 50% s and 50% p character. Examples of sp Hybridization: All compounds of beryllium like BeF 2, BeH 2, BeCl 2; All compounds of carbon-containing triple bond like C 2 H 2. sp 2 Hybridization
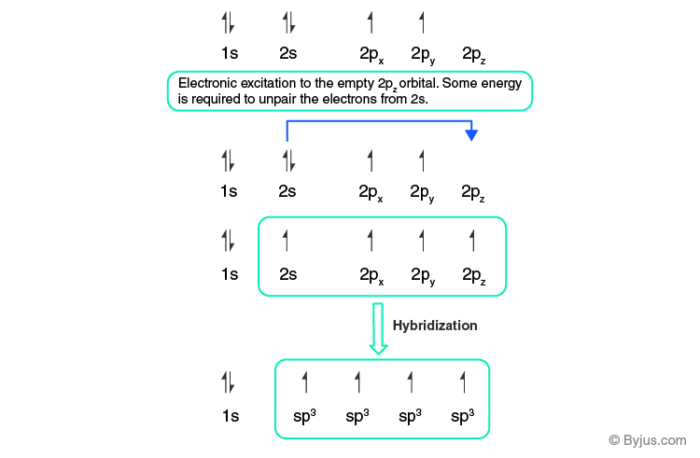
What is the hybridization of each carbon in this molecule? Explanation: This is because the first carbon has formed four bonds. So as you can see from the picture one electron from 2s orbital moves to the empty 2pz orbital. The 2s and the three 2p orbitals hybridise together and each orbital will be completed by adding one more electron from sharing with N, H, H, and the other C.
each carbon atom with the appropriate hybridization label chj hybridized hybndized chz hybrdized chz chj previous 8 give up view solution check answer aboutue a pmaicy policy tetms of use 62336
Hybridization - Chemistry Video | Clutch Prep Example #1: Determine the hybridization of the sulfur atom within SBr 4. Report issue. Practice: How many of the following molecules have sp 3 d 2 hybridization on the central atom? SeCl 6 XeCl 4 IF 5 AsCl 5. Practice: How many unhybridized orbitals does the beryllium atom ...
Solved Label each carbon atom with the appropriate | Chegg.com Label each carbon atom with the appropriate hybridization. sp,sp2 or sp3 Question : Label each carbon atom with the appropriate hybridization. sp,sp2 or sp3 This problem has been solved!
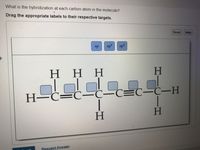
Answered: of 20 > Label each carbon atom with the… | bartleby Transcribed Image Text: of 20 > Label each carbon atom with the appropriate hybridization. sp hybridized CH3 Answer Bank sp hybridized sp hybridized ECH, sp hybridized sp hybridized at sp hybridized CH, sp hybridized lo Tv sp hybridized ČH3 an wit pai PrtScn Home End F10 PgUp F8 F9 Expert Solution Want to see the full answer?
OChem Spring 2017 Exam 1 Flashcards | Quizlet Label each carbon atom with the appropriate hybridization (cover bottom) ... Sapling Hw Ch 1.18. a) 120° b) 109.5° Label each carbon atom with its optimum C-C-C bond angle (cover bottom) Sapling Hw Ch 1.19. Rank the following compounds according to increasing positive character of the carbon atom CH₃F
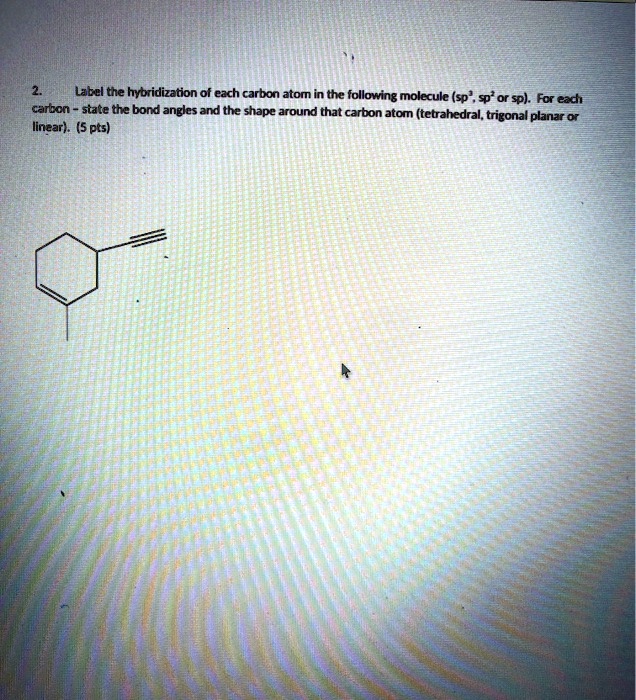
In the hydrocarbon (a) what is the hybridization at each carbon atom in ... In the hydrocarbon (a) what is the hybridization at each carbon atom in the molecule? (b) how many s bonds are there in the molecule? (c) how many p bonds? (d) identify all the 120° bond angles in the molecule. [section 9.6] 2 See answers Advertisement Advertisement
label each carbon atom with the appropriate hybridization 🚀To book a personalized 1-on-1 tutoring session:👉Janine The Tutorhttps://janinethetutor.com🚀More proven OneClass Services you might be interested in:👉One...
PDF The Hybridization Model of Atoms in Molecules - cpp.edu the carbon atoms, perpendicular to the plane of the page. On the other side of each carbon atom, 180o away from the other carbon atom, we can attach a simple hydrogen atom, using its 1s atomic orbital to overlap in a sigma bond along the bonding axis (a first bond is always sigma bond). C C σCC H C σCH C H σCH C C πCC πCC C C top and ...
Can you label each carbon atom with the appropriate hybridization .... C=C bonded C atoms are planar and sp2 hybridized. C-C bonded C are sp3 hybridized. In this compound one C=C present and hence both carbons are sp2 hybridized. Left all the C atoms have four single bond around each C atom. so left all the C atoms are sp3 hybridized. number of electrons pairs around sp3 hybridized C atoms = 4 and
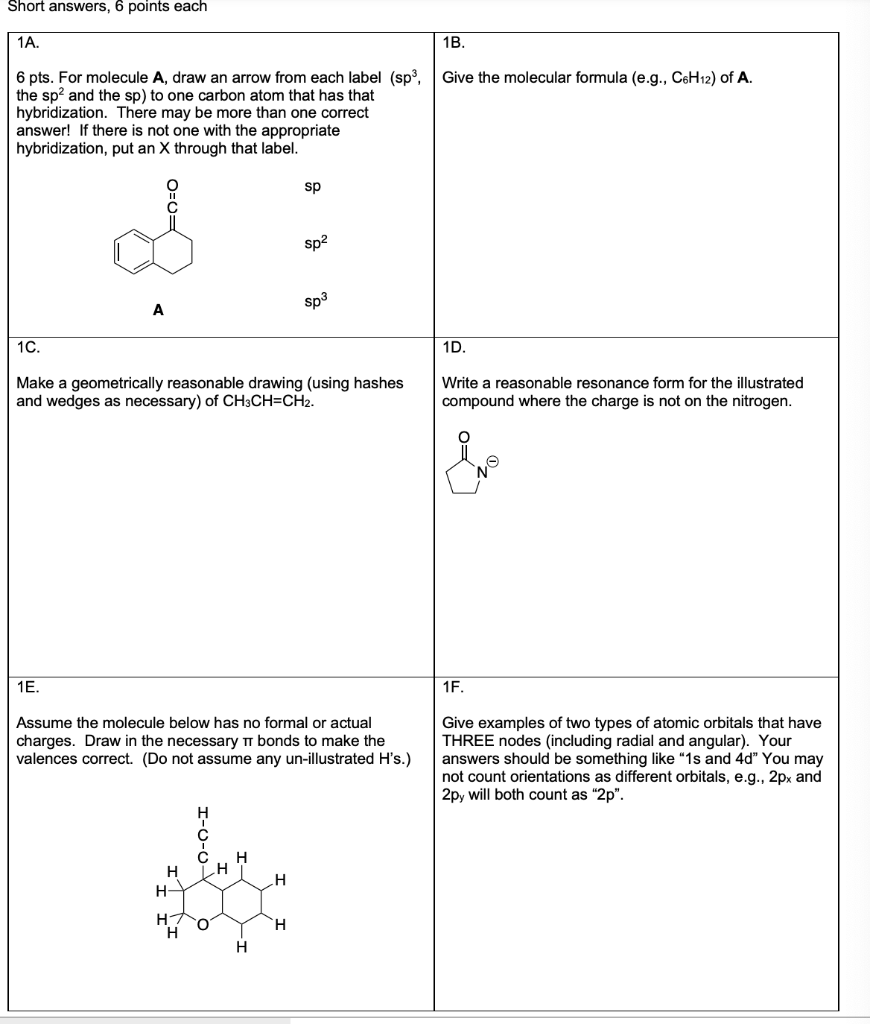

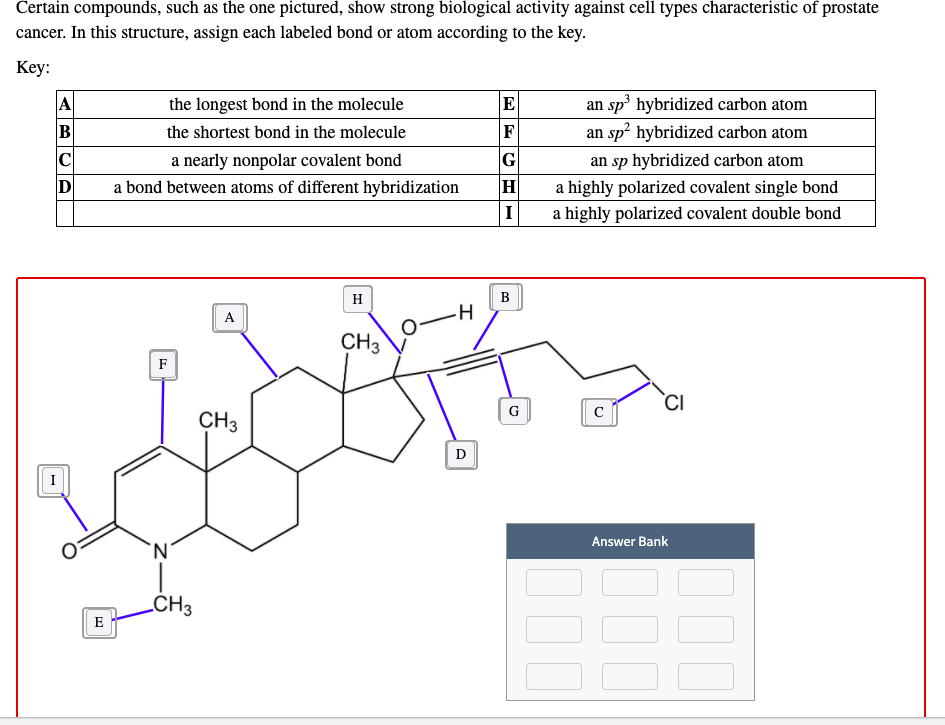
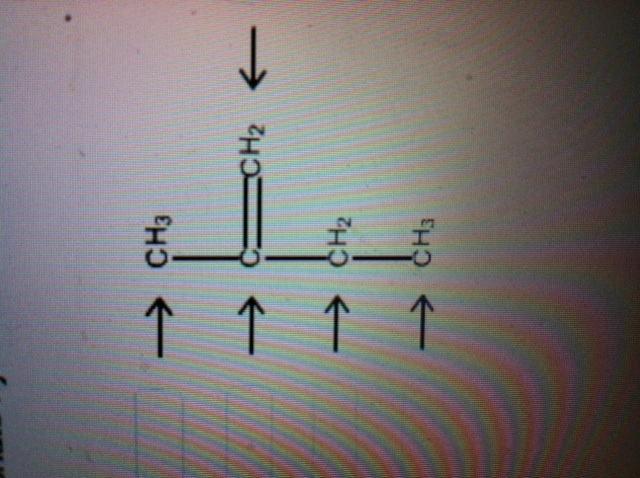

















Post a Comment for "44 label each carbon atom with the appropriate hybridization."