44 label each stereogenic center as r or s.
Unlocking P(V): Reagents for chiral phosphorothioate synthesis 02.08.2018 · Both (R)-PS– and (S)-PS–linked oligonucleotides can be readily accessed through selection of the desired ψ reagent enantiomer, (+)-ψ and (–)-ψ, respectively ().Derived from limonene, an inexpensive chiral pool terpene and common food additive, these OTP sulfide reagents are produced after epoxidation and ring opening. R/S - Two Stereogenic Centers R/S Naming Diastereoisomerism Meso Compounds Today, we'll look at naming compounds with stereocenters, and then we'll examine the complications which arise when a molecule has more than one stereocenter in it. First, though, let's look at a property in which one enantiomer differs from another. Enantiomers are alike in all respects but one.
A European Journal: EarlyView - Chemistry Europe A general copper-catalyzed carbonylative cross-coupling between amines and alkyl iodides based on a simple combination of catalytic CuCl and PMDETA in the presence of NaOH is reported. Primary, secondary and tertiary alkyl iodides and primary, cyclic and acyclic secondary amines as well as anilines were all shown to be suitable reaction partners, affording the corresponding …
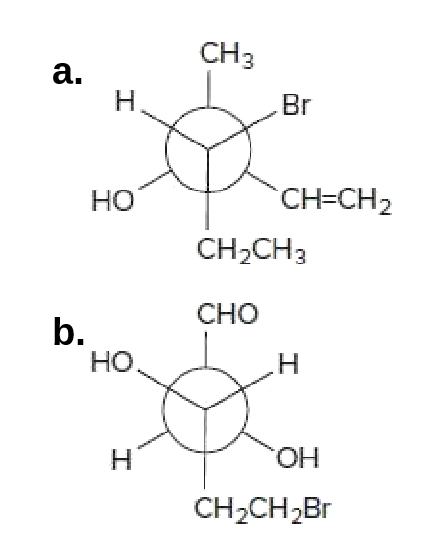
Label each stereogenic center as r or s.
5.6: Labeling Stereogenic Centers with R or S Stereocenters are labeled R or S The "right hand" and "left hand" nomenclature is used to name the enantiomers of a chiral compound. The stereocenters are labeled as R or S. Consider the first picture: a curved arrow is drawn from the highest priority ( 1) substituent to the lowest priority (4) substituent. Solved: Label each stereogenic center as R or S. | Chegg.com Organic Chemistry (4th Edition) Edit edition Solutions for Chapter 28 Problem 3P: Label each stereogenic center as R or S. … Solutions for problems in chapter 28 1P Label the stereogenic centers in each compound. - Numerade now here. The question is level this to eugenics centers in each morning. Q. In gives off a means even if all the three groups are different. Amines are not having that student exchange in their Norris energy because the in shimmers, which are middle images off each other, are inter convertible. The, uh please these heir to a means flips inside out, passing through a travel planner position ...
Label each stereogenic center as r or s.. Chiral drugs - Wikipedia In this approach: identify the chiral center, label the four atoms directly attached to the stereogenic center in question, assign priorities according to the sequence rule ( from 1 to 4), rotate the molecule until the lowest priority (number 4) substituent is away from the observer/viewer, draw a curve from number 1 to number 2 to number 3 ... Chiral drugs - Wikipedia Each twin is called an enantiomer. ... In this approach: identify the chiral center, label the four atoms directly attached to the stereogenic center in question, assign priorities according to the sequence rule ( from 1 to 4), rotate the molecule until the lowest priority (number 4) substituent is away from the observer/viewer, draw a curve from number 1 to number 2 to number 3 … Answered: Q2. Label all stereogenic centres as R… | bartleby Solution for Q2. Label all stereogenic centres as R or S. No explanation is required. H. CH3 CH2CH3 D INH2 N(CH3)2 H3CH2CO- Chapter 5 Assignment Saved 9 3 attempts l... - Physical Chemistry Chapter 5 Assignment Saved 9 3 attempts left Check my work Hint Be sure to answer all parts. 2 points Label each stereogenic center as R or S. CH3 CH(CH3)2 center 1 center 2 eBook Hic "CH3 HO SH References Stereocenter 1: (select) (select) Stereocenter 2: R S
Answered: Assign R, S designations to each… | bartleby Solution for Assign R, S designations to each stereogenic center in glucose. Rearrangement - Michigan State University Cyclic ketones have two alpha-carbon atoms, each of which might shift to the nascent 1º-carbocation. If R = H in the case shown here, these two groups are identical and on shifting give the same product. If R = CH 3, the 2º-alkyl group shifts preferentially, the chief product being 3-methylcyclohexanone; the 2-methyl isomer is a minor product ... Label Each Stereogenic Center As R Or S. - Modern Label Ideas The stereochemical labels r and s must be identical for each stereogenic center. If the substituents have multiple bonds the multiple bonded atoms are. If a molecule has a chiral center that is designated r the chiral center will be s in the molecules enantiomer. You need to be able to assign whether a chiral center is r or s. Answered: Tell whether the stereogenic centers… | bartleby Solution for Tell whether the stereogenic centers marked with an asterisk in the following structures have the R or the S configuration: CH, H. CO,H b. H2N- CH…
SOLVED:Label each stereogenic center as R or S. - Numerade let's label each stereo centers are s for part A iodine has the highest priority than the Ethel on the metal than hydrogen. But the lowest priority back, which is hydrogen. And if we move around the ring me of iodine, Ethyl carbon. This is the counter clockwise direction. Journal of the American Chemical Society | Vol 144, No 2 Shivaani S. Gandhi, Jesus I. Martinez Alvarado, and ; Abigail G. Doyle * Journal of the American Chemical Society 2022, 144, 2, 1045-1055 (Article) Publication Date (Web): January 5, 2022. Abstract; Full text; PDF; ABSTRACT. Organocatalytic Enantioselective Construction of Conformationally Stable C(sp 2)–C(sp 3) Atropisomers. Giulio Bertuzzi, Vasco Corti, Joseph A. … PDF Priority Rules for Naming Chiral Centers - The R,S System atomic number of the atom that is bonded directly to the chiral center. The higher the atomic number, the higher the priority.! Number the four atoms, or groups of atoms, such that "1" has the highest priority and "4" has the lowest priority. 2. If two or more of the atoms that are bonded directly to the chiral center are the same, then Answered: Label each stereogenic center as R or S… | bartleby The stereogenic center in the given compound has to be mentioned, Q: C) Label each asymmetric carbon in the compound below as R or S. H3C CH2CH2CI H3C CHO ⊙ Draw semicircle goes from 1→2→3 then check clockwise/anticlockwise rotation and 4th priority…
Journal of the American Chemical Society | Vol 144, No 2 Highly dispersed dual Au and Cu species enable efficient and selective photocatalytic conversion of methane to methanol and oxygenates on ZnO using O2 as the oxidant operated at ambient temperature, resulting in a nearly 100% selectivity and 14.1% apparent quantum yield at 365 nm. View the article.
Rearrangement - Michigan State University Were this to occur, both carbon substituents (1 R & 2 R) would be candidates for the subsequent 1,2-shift. In practice, however, it is always the group anti to the departing OH that migrates to nitrogen. This stereospecificity indicates that the 1,2-shift is concerted with N-O cleavage, as shown below. The resulting N-alkylated nitrilium intermediate will react with nucleophiles (e.g. …
Topic 5. Stereochemistry.pdf - Topic 5: Stereochemistry ... - Course Hero • This is done by adding the prefix R or S to the IUPAC name of the enantiomer. • To designate enantiomers as R or S, priorities must be assigned to each group bonded to the stereogenic center, in order of decreasing atomic number. • The atom of highest atomic number gets the highest priority (1).Labeling Stereogenic Centers with R or S
Molecules: Identifying Chiral Centers, Meso Compounds, and ... Dec 17, 2021 · A meso compound contains a plane of symmetry and so is achiral, regardless of whether the molecule has a chiral center. A plane of symmetry is a plane that cuts a molecule in half, yielding two halves that are mirror reflections of each other. By definition, a molecule that's not superimposable on its mirror image is a chiral molecule.
Chemistry Department May 9, 2009 Organic Chemistry 233 ... I. Circle the correct answer in each of the following: (10 pts) ¾ The number of stereogenic centers in the molecule below is: N H CH 3 NH 2 OH a. 1 b. 2 c. 3 d. 4 e. 5 ¾ The number of possible stereoisomers of: CH CH Cl HO2C CH2 CH2 CO2H Cl
OCHEM Stereochemistry chpt 5 Flashcards | Quizlet An unknown compound X has two stereogenic centers that each have the R configuration. The diastereomer(s) of X will have the R AND S & S AND R configurations at the stereogenic centers. Stereoisomers are isomers that differe in their 3-dimensional arrangement of atoms.
Lone pair - Wikipedia The repulsive force of the oxygen atom's lone pairs pushes the hydrogens further apart, until the forces of all electrons on the hydrogen atom are in equilibrium. This is an illustration of the VSEPR theory. Dipole moments. Lone pairs can contribute to a molecule's dipole moment. NH 3 has a dipole moment of 1.42 D.
Chap 5: Stereochemistry Flashcards - Quizlet Which of the following are steps for assigning R or S to a stereogenic center? a) Assign priorities from 1 to 4 to each group bonded to the stereogenic center. b) Trace a circle from priority group 3 -> 2 -> 1 c) Orient the molecule with the lowest priority group to the back d) Orient the molecule with the highest priority group to the back



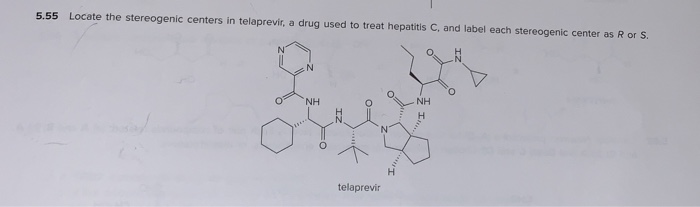

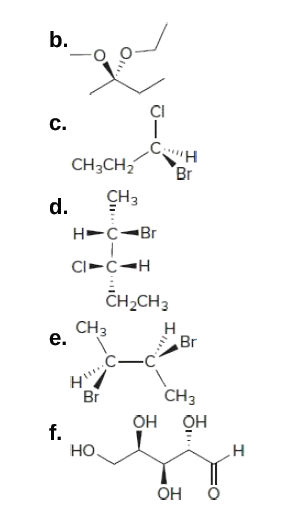
Post a Comment for "44 label each stereogenic center as r or s."